Oxidation of a double bond
1. KMnO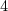
- mild conditions (vicinal diols)
- extreme conditions (carboxylic acids)
2. Ozonolysis
- mild conditions, reducing Zn/water environment (ketones/aldehydes)
- extreme conditions, oxidizing environment
3. NaBH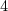 or LiAlH
or LiAlH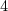
4. Peroxyacetic acids = CH CO
CO H or MCPBA (epoxide formation)
H or MCPBA (epoxide formation)
5. Hydroboration (NaBH , peroxide)
, peroxide)
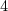
- mild conditions (vicinal diols)
- extreme conditions (carboxylic acids)
2. Ozonolysis
- mild conditions, reducing Zn/water environment (ketones/aldehydes)
- extreme conditions, oxidizing environment
3. NaBH
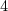 or LiAlH
or LiAlH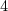
4. Peroxyacetic acids = CH
 CO
CO H or MCPBA (epoxide formation)
H or MCPBA (epoxide formation)5. Hydroboration (NaBH
 , peroxide)
, peroxide)Tags: rxns
Quelle:
Quelle:



