This flashcard is just one of a free flashcard set. See all flashcards!
74
The Three Laws of Thermodynamics
1. Energy cannot be created or destroyed:
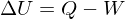
where U is the internal energy, Q is heat, W work.
2. There is no process who's sole effect is to transfer heat from a hot body to a cold body. Another way to put it is that no engine can convert energy from a heat reservoir entirely into mechanical energy.
Mathematically: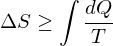
3. Entropy is zero at absolute zero.
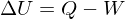
where U is the internal energy, Q is heat, W work.
2. There is no process who's sole effect is to transfer heat from a hot body to a cold body. Another way to put it is that no engine can convert energy from a heat reservoir entirely into mechanical energy.
Mathematically:
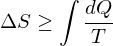
3. Entropy is zero at absolute zero.
Flashcard info:
Author: CoboCards-User
Main topic: Physics
Topic: GRE
School / Univ.: UC Santa Cruz
City: Santa Cruz, CA
Published: 20.10.2015

